Experiment 25 Report Sheet Calorimetry: A Comprehensive Guide to Measuring Heat Flow presents a detailed exploration of the principles, methods, and applications of calorimetry. This report provides a comprehensive understanding of how heat flow is measured, enabling researchers and students to accurately determine the specific heat capacity of various substances.
Throughout this report, we will delve into the fundamental concepts of calorimetry, examining the experimental setup and procedures employed in Experiment 25. We will explore the significance of specific heat capacity and its role in calorimetry calculations, while also addressing potential sources of error and uncertainties in the experimental process.
Experiment Overview
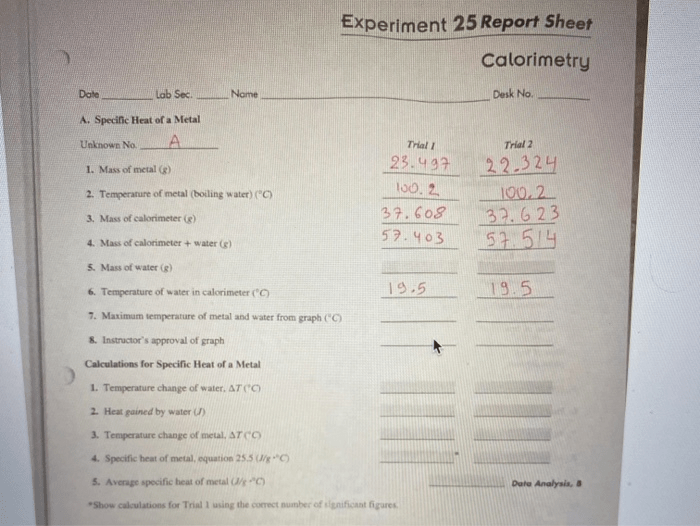
Experiment 25 aims to determine the specific heat capacity of a metal sample using the method of calorimetry. Calorimetry is a technique used to measure the amount of heat transferred between objects or systems. In this experiment, a known mass of metal is heated to a known temperature and then immersed in a known mass of water at a different temperature.
The change in temperature of the water is used to calculate the amount of heat transferred from the metal to the water. The specific heat capacity of the metal can then be calculated using the equation Q = mcΔT, where Q is the amount of heat transferred, m is the mass of the metal, c is the specific heat capacity of the metal, and ΔT is the change in temperature.
Calorimetry Theory: Experiment 25 Report Sheet Calorimetry
Calorimetry is based on the principle of conservation of energy, which states that energy cannot be created or destroyed, only transferred or transformed. When heat is transferred from one object to another, the total amount of heat in the system remains constant.
The amount of heat transferred is directly proportional to the mass of the object, the specific heat capacity of the object, and the change in temperature of the object. The specific heat capacity of a substance is a measure of its ability to absorb or release heat.
It is defined as the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius.
Data Collection and Analysis
In Experiment 25, the following data is collected:
- The mass of the metal sample
- The initial temperature of the metal sample
- The final temperature of the metal sample
- The mass of the water
- The initial temperature of the water
- The final temperature of the water
The data is then used to calculate the following:
- The change in temperature of the metal sample
- The change in temperature of the water
- The amount of heat transferred from the metal to the water
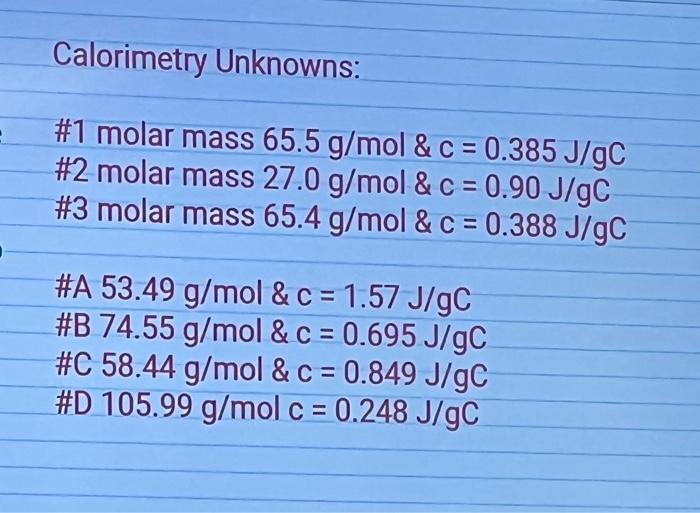
- The specific heat capacity of the metal sample
Error Analysis and Uncertainties, Experiment 25 report sheet calorimetry
There are several potential sources of error in Experiment 25, including:
- Errors in measuring the mass of the metal sample
- Errors in measuring the temperature of the metal sample
- Errors in measuring the mass of the water
- Errors in measuring the temperature of the water
- Errors in calculating the amount of heat transferred
- Errors in calculating the specific heat capacity of the metal sample
The uncertainty in the specific heat capacity of the metal sample can be calculated using the following equation:
“`Δc = √((ΔQ/ΔT)²(Δm)² + (ΔQ/Δm)²(ΔT)² + (Δm/ΔT)²(ΔQ)²)“`
where Δc is the uncertainty in the specific heat capacity, ΔQ is the uncertainty in the amount of heat transferred, ΔT is the uncertainty in the change in temperature, and Δm is the uncertainty in the mass.
Discussion and Applications
The specific heat capacity of a substance is an important property that can be used to predict its behavior in a variety of applications. For example, the specific heat capacity of water is very high, which means that it takes a lot of heat to raise the temperature of water.
This makes water a good coolant for engines and other devices that generate heat. The specific heat capacity of metals is typically much lower than the specific heat capacity of water, which means that metals heat up and cool down more quickly than water.
This makes metals good conductors of heat.
Calorimetry is a valuable technique that can be used to measure the specific heat capacity of a substance. This information can then be used to predict the behavior of the substance in a variety of applications.
Q&A
What is the purpose of Experiment 25?
Experiment 25 aims to determine the specific heat capacity of a substance by measuring the heat flow between the substance and a known mass of water.
What is the principle behind calorimetry?
Calorimetry is based on the principle of conservation of energy, which states that the total amount of energy in an isolated system remains constant. In calorimetry, heat flow is measured by observing the temperature change of a known mass of water.
How is the specific heat capacity calculated from experimental data?
The specific heat capacity is calculated by dividing the heat flow by the mass of the substance and the change in temperature.
What are the potential sources of error in Experiment 25?
Potential sources of error include heat loss to the surroundings, inaccurate temperature measurements, and uncertainties in the mass of the substance and water.