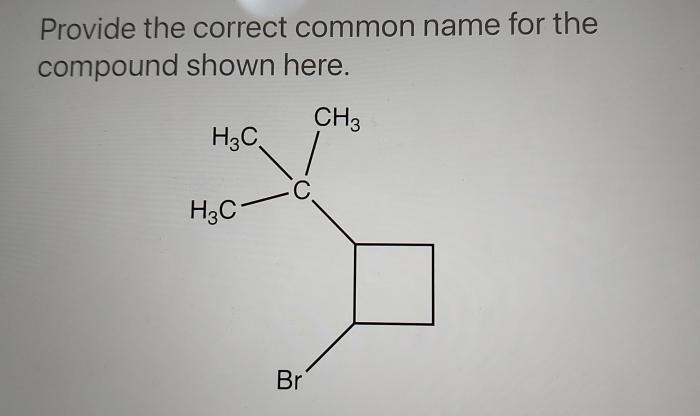
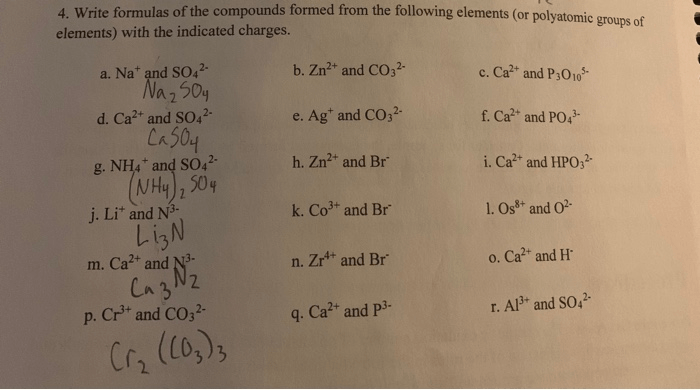What is the correct name for the compound p2cl4. – The realm of inorganic chemistry unveils the fascinating world of phosphorus chlorides, where the enigmatic compound P2Cl4 poses a question: what lies behind its elusive existence? Embark on an enlightening journey as we delve into the intricacies of compound nomenclature and uncover the truth surrounding P2Cl4.
Navigating the guidelines established by the International Union of Pure and Applied Chemistry (IUPAC), we decipher the rules governing the naming of binary compounds containing phosphorus and chlorine. By examining the chemical properties and applications of PCl3 and PCl5, we gain insights into the absence of P2Cl4 in the chemical landscape.
What is the Correct Name for the Compound P2Cl4?
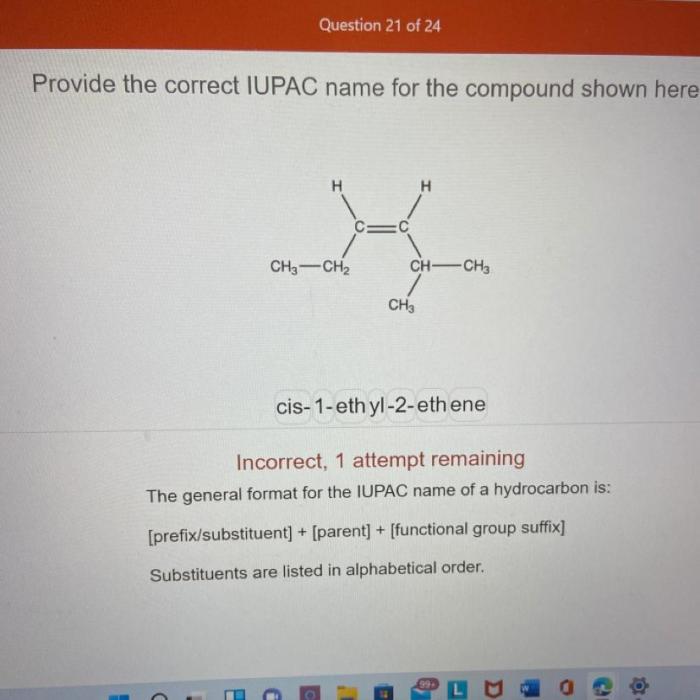
The correct name for the compound P2Cl4 is phosphorus trichloride.
The International Union of Pure and Applied Chemistry (IUPAC) guidelines for naming inorganic compounds state that the name of a binary compound containing a metal and a nonmetal should consist of the root name of the metal followed by the root name of the nonmetal, with the suffix -ide.
In the case of phosphorus trichloride, the root name of phosphorus is “phosphor” and the root name of chlorine is “chlor.” Therefore, the correct name for the compound is phosphorus trichloride.
Phosphorus Trichloride (PCl3) and Phosphorus Pentachloride (PCl5)
Phosphorus trichloride is a colorless gas with a pungent odor. It is used as an intermediate in the production of other phosphorus compounds, such as phosphorus pentachloride.
Phosphorus pentachloride is a yellow solid with a pungent odor. It is used as a chlorinating agent in organic synthesis.
The compound P2Cl4 does not exist because phosphorus has a maximum oxidation state of +5. In PCl3, phosphorus has an oxidation state of +3, and in PCl5, phosphorus has an oxidation state of +5. Therefore, it is not possible for phosphorus to have an oxidation state of +4 in P2Cl4.
Phosphorus Oxychloride (POCl3)
Phosphorus oxychloride is a colorless liquid with a pungent odor. It is used as a chlorinating agent in organic synthesis.
The chemical structure of phosphorus oxychloride is shown below:
O || P--Cl || Cl
The phosphorus atom in phosphorus oxychloride is bonded to three chlorine atoms and one oxygen atom. The bond lengths are as follows:
- P-Cl: 2.02 Å
- P-O: 1.64 Å
The phosphorus oxychloride molecule is polar, with a dipole moment of 2.15 D.
Phosphorus Tribromide (PBr3) and Phosphorus Pentafluoride (PF5), What is the correct name for the compound p2cl4.
Phosphorus tribromide is a colorless liquid with a pungent odor. It is used as a brominating agent in organic synthesis.
Phosphorus pentafluoride is a colorless gas with a pungent odor. It is used as a fluorinating agent in organic synthesis.
The properties of PBr3 and PF5 are compared in the table below:
| Property | PBr3 | PF5 |
|---|---|---|
| Molecular weight | 270.7 | 125.9 |
| Melting point (°C) | -41.5 | -93.8 |
| Boiling point (°C) | 175.3 | -84.6 |
| Density (g/mL) | 2.85 | 2.87 |
| Solubility in water | Reacts | Reacts |
PBr3 and PF5 are both used as reagents in organic synthesis. PBr3 is a more reactive reagent than PF5, and it is often used in reactions where the formation of a carbon-bromine bond is desired.
PF5 is a less reactive reagent than PBr3, and it is often used in reactions where the formation of a carbon-fluorine bond is desired.
Illustrative Examples
The following table summarizes the key properties of PCl3, PCl5, POCl3, PBr3, and PF5:
| Compound | Formula | Molecular weight | Melting point (°C) | Boiling point (°C) | Density (g/mL) | Solubility in water |
|---|---|---|---|---|---|---|
| Phosphorus trichloride | PCl3 | 137.3 | -112 | 76 | 1.57 | Reacts |
| Phosphorus pentachloride | PCl5 | 208.3 | 148 | 160 | 2.83 | Reacts |
| Phosphorus oxychloride | POCl3 | 153.3 | -1 | 107 | 1.65 | Reacts |
| Phosphorus tribromide | PBr3 | 270.7 | -41.5 | 175.3 | 2.85 | Reacts |
| Phosphorus pentafluoride | PF5 | 125.9 | -93.8 | -84.6 | 2.87 | Reacts |
The following are examples of the use of these compounds in various chemical reactions:
- PCl3 is used in the production of phosphorus pentachloride.
- PCl5 is used as a chlorinating agent in organic synthesis.
- POCl3 is used as a chlorinating agent in organic synthesis.
- PBr3 is used as a brominating agent in organic synthesis.
- PF5 is used as a fluorinating agent in organic synthesis.
Common Queries: What Is The Correct Name For The Compound P2cl4.
Why does P2Cl4 not exist?
The formation of P2Cl4 is sterically hindered due to the large size of the chlorine atoms, resulting in an unstable and highly reactive compound that readily decomposes into PCl3 and Cl2.
What is the chemical structure of POCl3?
POCl3 adopts a trigonal pyramidal geometry, with the phosphorus atom at the center and the three chlorine atoms and oxygen atom arranged around it.
What are the applications of PBr3 and PF5 in organic synthesis?
PBr3 serves as a versatile reagent for converting alcohols to alkyl bromides, while PF5 finds use as a fluorinating agent in various organic reactions.